We’ll teach you everything you need to know—whether you like or not.
Videos, written explanations, answer-choice snippets, low-res summaries, stimulus highlighting…you probably don’t know what half that stuff means, but you’ll thank us for it later.
12+ classes per weekday. 60+ per week. 3,000+ recorded classes—ready when you are.
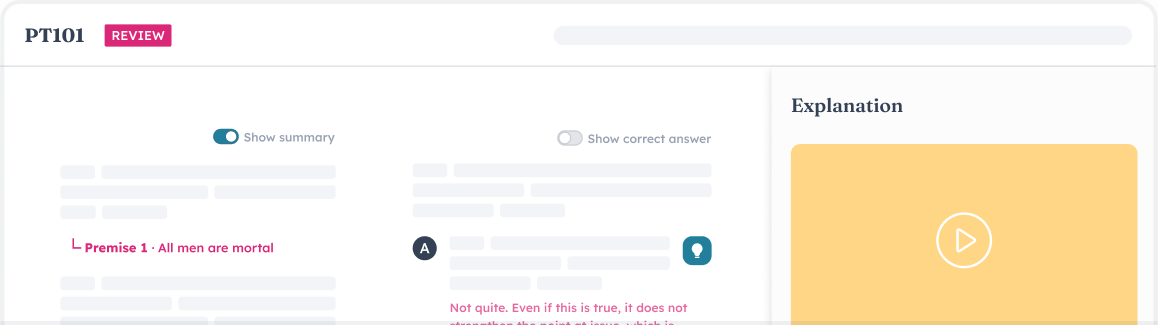
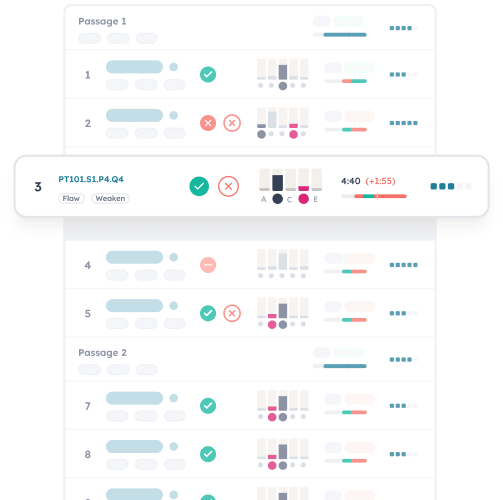
Outwork and outsmart the LSAT with insanely granular test analytics.
Reveal the practice areas that are worth the most for you and feel the confidence of data-driven study planning.
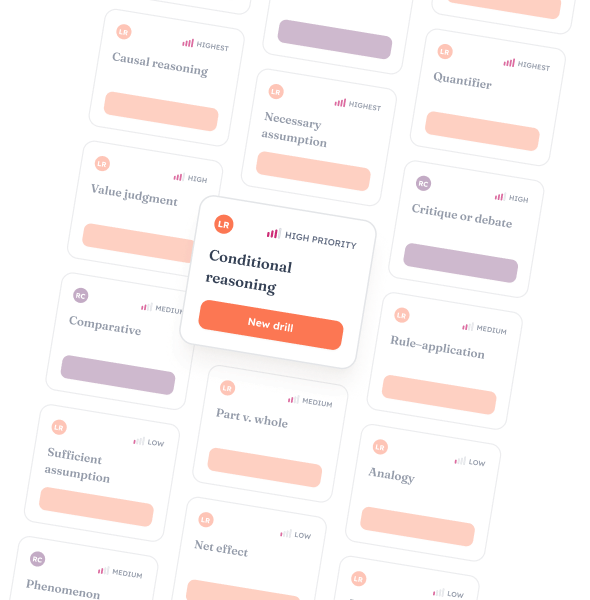
Targeted drills turn discipline into points.
Practice the questions you hate until they become the questions you dominate.


Based on an automated sentiment analysis of Reddit posts mentioning 7Sage, we counted 7,500 upvotes on 336 threads. We think this is a (very) conservative estimate— but we’ll leave it to you to scour the subs and see why so many of our students come from Reddit.
LSAC requires every student who enrolls in a prep course to purchase a LawHub Advantage subscription for $120/year. The fee goes to LSAC, not 7Sage.
LSAC requires every student who enrolls in a prep course to purchase a LawHub Advantage subscription for $120/year. The fee goes to LSAC, not 7Sage.
LSAC requires every student who enrolls in a prep course to purchase a LawHub Advantage subscription for $120/year. The fee goes to LSAC, not 7Sage.
| Compare plans | Core |
Live
|
Coach |
|
Video and text explanations
|
All official LSATs | All official LSATs | All official LSATs |
|
Adaptive study scheduler
|
|||
|
Comprehensive video curriculum
|
|||
|
Analytics & customizable drills
|
|||
|
Discussion forum & study groups
|
|||
|
Import LawHub PrepTests
|
|||
|
Daily live classes
|
|||
|
Class recordings
|
|||
|
Ask a tutor
|
|||
|
Private tutoring
|
|||
|
Personalized accountability emails
|
|||

